Beam Therapeutics’ BEAM-302: A Breakthrough in AATD Treatment
Beam Therapeutics Achieves Milestone in AATD Treatment with BEAM-302
Beam Therapeutics has announced groundbreaking initial data from its Phase I/II trial of BEAM-302, a gene-editing therapy for alpha-1 antitrypsin deficiency (AATD). The results demonstrate the first-ever clinical genetic correction of the disease-causing mutation in AATD patients, potentially setting a new benchmark for efficacy in this therapeutic area [1] [3].
Understanding AATD and BEAM-302
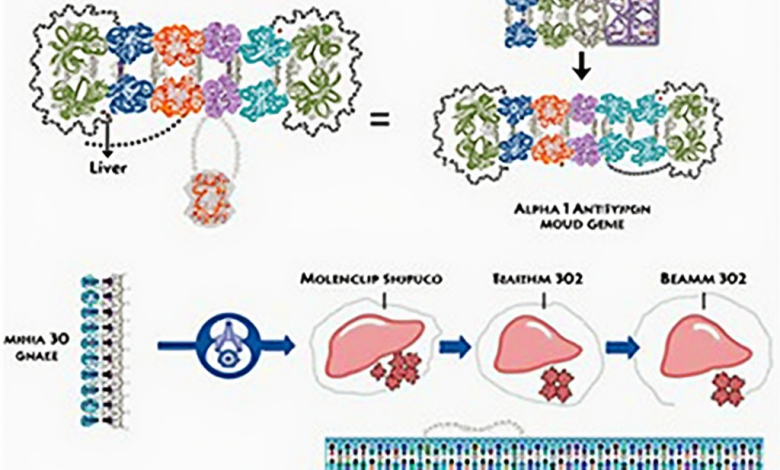
Alpha-1 antitrypsin deficiency (AATD) is a rare genetic condition affecting approximately 1 in 2,500 people. It is characterized by insufficient expression of the alpha-1 antitrypsin (AAT) protein, which plays a crucial role in protecting the lungs and liver from damage. When AAT levels are low, it can lead to serious lung diseases, such as emphysema, and liver complications, including cirrhosis. BEAM-302, developed by Beam Therapeutics, is a liver-targeted formulation of base-editing agents that aim to correct the most common genetic mutation associated with AATD. By effectively increasing functional AAT levels and reducing the accumulation of the mutant protein, BEAM-302 addresses the underlying cause of this debilitating condition.
Promising Efficacy Results
The Phase I/II trial results for BEAM-302 have shown remarkable efficacy:
– Total AAT levels increased significantly from 4.4 µM at baseline to 12.4 µM at 28 days post-treatment with a 60-mg dose.
– The therapy has successfully reduced circulating concentrations of the mutant AAT protein by an impressive 78% in patients treated at the 60-mg dose level.
– These encouraging results demonstrate a sustainable therapeutic effect that surpasses the critical efficacy threshold of 11 µM, which is essential for effective disease management.
These results signify a pivotal advancement in the treatment of AATD, indicating that BEAM-302 may provide a definitive therapeutic option for patients suffering from this condition.
Safety Profile
In addition to its efficacy, BEAM-302 has demonstrated an acceptable safety profile:
– The therapy was well-tolerated among trial participants, with only mild to moderate toxicities reported.
– Importantly, no serious adverse events or dose-limiting toxicities have been observed during the study, reinforcing the therapy’s safety.
– A small number of patients experienced Grade 1 liver enzyme elevations, but these were asymptomatic and did not require any clinical intervention, suggesting that the treatment is generally safe for use.
This favorable safety profile is crucial for patient acceptance and future regulatory approvals.
Market and Competitive Landscape
The market for AATD treatments is rapidly evolving, and BEAM-302 appears to position Beam Therapeutics favorably among current competitors:
– Analysts at William Blair have emphasized that the data from the BEAM-302 trial validates Beam’s innovative platform and may establish a new efficacy benchmark in the AATD treatment landscape.
– For comparison, Wave Life Sciences’ RNA editing approach showed AAT levels of 10.8 µM in a separate trial conducted in October 2024.
– The unique advantage of BEAM-302 lies in its potential as a one-time treatment, which could offer a more durable solution compared to the transient effects observed with RNA editing techniques.
As the competitive landscape for AATD therapies becomes increasingly crowded, BEAM-302’s demonstrated efficacy and safety profile could give Beam Therapeutics a strategic edge.
Implications and Future Outlook
The implications of these promising results are significant not only for patients but for the broader field of gene therapy:
– Beam Therapeutics foresees the possibility of an expedited regulatory pathway, with the potential for accelerated FDA approval based on the compelling trial outcomes.
– Despite the positive results, the stock market response has shown mixed signals; Beam’s stock price dropped by 9% following the announcement, revealing investor concerns about the long-term efficacy of the treatment [5].
– The next steps involve extensive monitoring of long-term efficacy and safety as the trial progresses.
– If positive results continue, BEAM-302 could realistically become the new standard of care in the treatment of AATD, transforming patient outcomes.
A New Era in Genetic Medicine
The initial data from the BEAM-302 trial represents a landmark achievement in the treatment of alpha-1 antitrypsin deficiency. By demonstrating the first-ever clinical genetic correction of the disease-causing mutation, Beam Therapeutics has paved the way for new possibilities in gene-editing therapies. The ability of BEAM-302 to increase functional AAT levels and reduce mutant protein accumulation directly addresses the root cause of AATD, offering a more effective and durable treatment option for affected patients.
While the results are indeed promising, it is essential to await long-term efficacy and safety data to fully understand the therapy’s potential impact. The mixed market response underscores the need for ongoing research and development efforts to validate BEAM-302 as a transformative therapy. As the trial advances, the medical community and investors alike will be closely monitoring for sustained benefits and emerging safety signals.
If BEAM-302 continues to deliver positive results in larger patient populations over extended periods, it could revolutionize the treatment landscape for AATD and serve as a model for addressing other genetic disorders. This breakthrough not only represents hope for patients grappling with AATD but also validates the immense potential of base editing as a powerful tool in the expansive field of genetic medicine [6].




